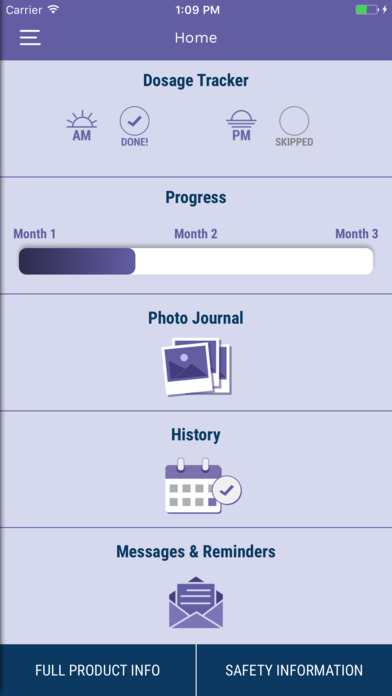
Facing Forward by Cutanea
The Facing Forward app is a mobile application to help track your acne treatment. It offers features like: daily reminders, photo journal, dosing calendar, information for healthcare providers and pharmacy information.
INDICATIONS AND USAGE
AKTIPAK™ (erythromycin and benzoyl peroxide) Gel, 3%/5% is indicated for the topical treatment of acne vulgaris.
IMPORTANT SAFETY INFORMATION
Contraindications: AKTIPAK™ is contraindicated in those individuals who have shown hypersensitivity to any of its components.
Precautions: For topical use only; not for ophthalmic use. Concomitant topical acne therapy should be used with caution because a possible cumulative irritancy effect may occur, especially with the use of peeling, desquamating or abrasive agents. If severe irritation develops, discontinue use and institute appropriate therapy. The use of antibiotic agents may be associated with the overgrowth of nonsusceptible organisms. If this occurs, discontinue use and take appropriate measures. Avoid contact with eyes and all mucous membranes.
Pregnancy: It is not known whether AKTIPAK™ can cause fetal harm when administered to a pregnant woman. AKTIPAK™ should be given to a pregnant woman only if clearly needed. It is not known whether the ingredients of AKTIPAK™ are excreted in human milk. Therefore, caution should be exercised when erythromycin is administered to a nursing woman.
Pediatric Use: The safety and effectiveness of AKTIPAK™ in pediatric patients below 12 years of age have not been established.
Adverse Reactions: The most frequently reported adverse events reported in clinical trials include: dry skin, application site reaction, blepharitis, pruritus, and photosensitivity.
Please see accompanying full Prescribing Information and Important Safety Information. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit http://www.fda.gov/Safety/MedWatch/default.htm or call 1-800-FDA-1088.
For full Product Information, please visit http://www.aktipak.com/wp-content/themes/Aktipak/img/pdf/CUTA_11042_AKT-WEBSITE_DV1_021717_Desktop_FULL_PI.pdf